TRANSPORT IN PLANTS
POINTS TO REMEMBER :
Methods of Facilitated Diffusion :
- Translocation : Transport of substances in plants over longer distances through the vascular tissue (Xylem and Phloem) is called translocation.
- Means of transport : The transport of material into and out of the cells is carried out by a number of methods. These are diffusion, facilitated diffusion and active transport.
- Diffusion : Diffusion occurs from region of higher concentration to region of lower concentration across the permeable membrane. It is passive and slow process. No energy expenditure takes place.
- Facilitated diffusion : The diffusion of hydrophilic substances along the concentration gradient through fixed membrane transport protein without involving energy expenditure is called facilitated diffusion. For this the membrane possesses aquarporins and ion channels. No energy is utilized in this process.
Active transport :
- Some carrier or transport proteins allow diffusion only if two types of molecules moves together.
- Symport: both molecules cross the membrane in the same direction.
- Antiport: both molecule moves in opposite direction.
- Uniport: one type of molecule moves across the cell membrane.
Water potential :
- Active transport is carried by the movable carrier proteins (pumps) of membrane.
- Active transport uses energy to pump molecules against a concentration gradient from a low concentration to high concentration (uphill-transport).
- It is faster than passive transport.
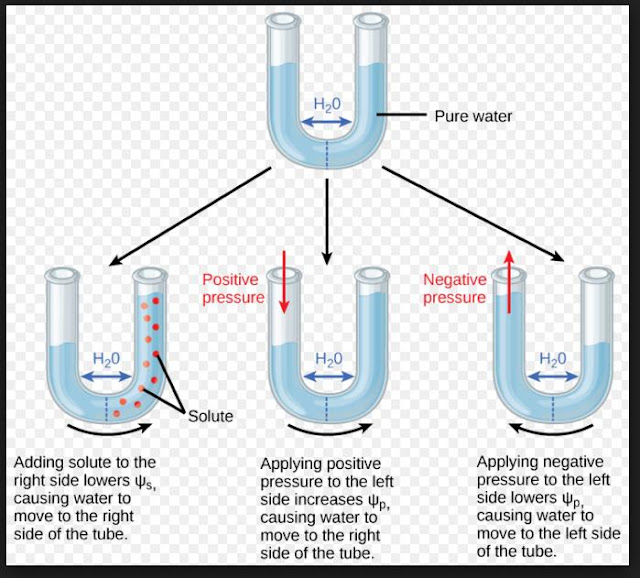
Osmosis :
- Water molecule possesses kinetic energy.
- The greater the concentration of water in a system, the greater is its kinetic energy or water potential.
- Pure water has the highest water potential.
- Water always moves from higher water potential to lower water potential.
- Water potential is denoted by Ψw (Psi) and measured in Pascals (Pa). The water potential of a cell is affected by solute potential (Ψs) and pressure potential (Ψp).
- Ψ w = Ψ s + Ψ p
- Water potential of pure water at standard temperature which is not under any pressure is taken to be zero (by convention).
- Osmosis is movement of solvent or water molecules from the region of their higher diffusion pressure or free energy to the region of their lower diffusion pressure or free energy across a semi-permeable membrane.
- Water molecules move from higher water potential to lower water potential until equilibrium is reached.
- Study this figure in which the two chambers, A and B, containingsolutions are separated by a semi-permeable membrane.
- (a)Solution of which chamber has a lower water potential
- (b)Solution of which chamber has a lower solute potential?
- (c)In which direction will osmosis occur?
- (d)Which solution has a higher solutepotential?
- (e)At equilibrium which chamber willhave lower water potential?
- (f)If one chamber has a Ψ Ψ Ψ Ψ Ψ of – 2000kPa, and the other – 1000 kPa, whichis the chamber that has the higherΨΨΨΨΨ?
- (g)What will be the direction of themovement of water when twosolutions with Ψw = 0.2 MPa andΨw = 0.1 MPa are separated by aselectively permeable membrane?
Long distance transport of water :Plasmolysis :
Some examples of Imbibition :
- Process of shrinkage of protoplasm in a cell due to exosmosis in hypertonic solution.
- Turgor pressure: a plant cell placed in hypotonic solution, water enters into it due endosmosis and the cytoplasm exert pressure against the cell wall called turgor pressure.
- Imbibition: Imbibition is the phenomenon of adsorption of water or any other liquid by the solid particles of a substance without forming a solution.
- If a dry piece of wood is placed in water, it swells and increases in its volume.
- If dry gum or pieces of agar-agar are placed in water, they swell and their volume increases.
- When seeds are placed in water they swell up.
- Mass flow: Mass flow is the movement of substances (water, minerals and food) in bulk from one point to another as a result of pressure differences between two points.
- Translocation: the bulk movement of substance through the conducting or vascular tissue is called translocation.
How do plants absorb water?
- Transport of water in plants: Water is absorbed by root hairs, then water moves upto xylem by two pathways − apoplast and symplast pathway.
- Apoplast pathway :
- Movement of water takes place exclusively through the intercellular spaces and the walls of the cells.
- Movement through the apoplast does not involve crossing the cell membrane.
- Movement depends on the gradient.
- The apoplast does not provide any barrier to water movement.
- Water movement is trough mass flow.
- Symplast pathway :
- System of interconnected protoplasts.
- Neighboring cells are connected through cytoplasmic strands that extend through plasmodesmata.
- Water enters into the cytoplasm by crossing the plasma membrane.
- Intercellular movement is through the plasmodesmata.
- Casparian strip : endodermis is impervious to water because of a band of suberised matrix called casparian strip.
Water movement up a plant :
- Root pressure : A hydrostatic pressure existing in roots which push the water up in xylem vessels.
- Guttation : The water loss in its liquid phase at night and early morning through special openings of vein near the tip of leaves.
- Transpiration pull : The transport of water to the tops of trees occurs through xylem vessels. The forces of adhesion and cohesion maintain thin and unbroken columns of water in the capillaries of xylem vessels through which it travels upward. Water is mainly pulled by transpiration from leaves. (Cohesion-tension-transpiration pull Model)
- Transpiration : The loss of water through stomata of leaves and other aerial parts of plants in form of water vapour.
- Transpiration driven ascent of xylem sap depends on the following physical properties of water:
- Cohesion : mutual attraction between water molecules.
- Adhesion : attraction of water molecules to polar surface(such as the surface of tracheary elements)
- Surface tension : water molecules are attracted to each other in the liquid phase more than to water in the gas phase.
Role of transpiration :
- Creates transpiration pull for absorption and transport of plants.
- Supplies water for photosynthesis.
- Transports minerals from the soil to all parts of the plants.
- Cools leaf surfaces, sometimes 10 to 15 degrees, by evaporative cooling.
- Maintains the shape and structure of the plants be keeping cells turgid.
Factors affecting transpiration : Temperature, light, humidity, wind speed, number and distribution of stomata, water status of plant.
Uptake and transport of mineral nutrients :
- Ions are absorbed by the roots by passive and active transport.
- The active uptake of ions requires ATP energy.
- Specific proteins in membranes of root hair cells actively pump ions from the soil into the cytoplasm of epidermal cells and then xylem.
- The further transport of ions to all parts of the plant is carried through the transpiration stream.
- The glucose is prepared at the source by the process of photosynthesis and is converted to sucrose (sugar).
- This sugar is then moved into sieve tube cells by active transport. It produces hypertonic condition in phloem.
- Water in the adjacent xylem moves into phloem by osmosis.
- Due to osmotic (turgor) pressure, the phloem sap moves to the areas of lower pressure.
- At the sink, osmotic pressure is decreased.
- The incoming sugar is actively transported out of the phloem and removed as complex carbohydrates (sucrose).
- As the sugar is removed, the osmotic pressure decreases, the water moves out of the phloem and returns to the xylem.
Expert NEET/AIIMS Medical Biology Faculty Kota.